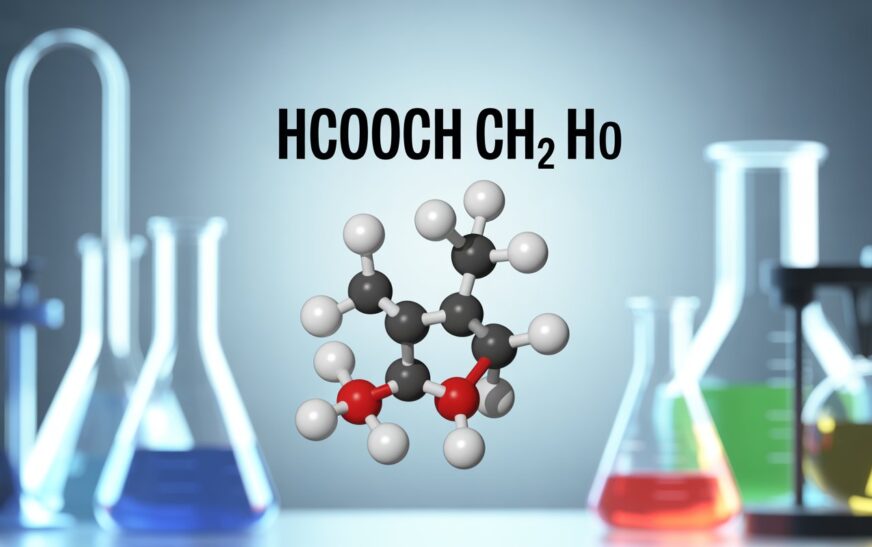
A complex molecule can be referred to by a concise formula often, which is the case with the keyword hcooch ch2 h2o, which demonstrates a complex combination of functional groups, thus can be discussed in aspects of both structure, reactivity, and significance in organic chemistry. Although one might first be tempted into thinking the notation is odd, its deconstruction can give one helpful insight into chemical bonding as well as possible applications. In the article we will be dealing with what hcooch ch2 h2o means, it is significant in the study of chemicals and its relation to a practical area of life like organic synthesis and pharmaceuticals and environmental science.
Analysis of the Formula
In order to comprehend hcooch ch2 h2o, we should draw a distinction between the two parts:
- HCOOCH – This seems like a formate ester structure where the HCOO- is the formyl or formate and CH is attached to a carbon. Esters are also widespread in organic chemistry, and even related to odours, flavours and solvents.
- CH2 CH 2 – methylene group is a flexible unit in organic chemistry. It occurs as a building block in hydrocarbons, polymers, and biomolecules.
- H2O- Water as the universal solvent Water is an essential component of practically every chemical and biologic process. Its addition to this expression could imply hydration, hydrolysis or active course of solvent treatment with the other groups.
- Added together, hcooch ch2 h2o can be discerned as a chemical illustration of a compound or a reaction in which a formate ester reacts with molecules of methylene and water.
The hcooch ch2 h2o is chemically important.
The keyword hcooch ch2 h2o indicates reactions implying the formation of formates, hydrocarbons and water. We shall see how it may be chemically pertinent:
- Formates (HCOO – derivatives): Formates occur widely in industries and play an important role in the synthesis of formic acid and other esters. These also exist in the natural metabolic pathways.
- Methylene (CH 2): This group plays a major role in chain formation and connects the units of the organic molecules. It offers rigidity and flexibility to compounds.
- Water (H 2 O): a very important reagent and solvent in Hydrolysis and Condensation reactions. In ester chemistry, hydrolysis is commonly a reaction that happens in the presence of water to make ester derivatives by breaking them into acids and alcohols.
Consequently, hcooch ch2 h2o can represent the hydrolysis of ester or another similar change in a chemical composition of organic chemistry.
Industrial and Lab Relevance
The knowledge of compounds that are written down as hcooch ch2 h2o is critical in a number of disciplines:
- Pharmaceuticals: A variety of medicines are stabilized and controlled release dependent on ester structure. Metabolism of some medications: Hydrolysis is a process in which water (H 2 O) reacts with esters to activate some medications in the body.
- Biochemistry: Esters and methylene are the key ones in the biological intermediates. The hcooch ch2 h2o Hydrolytic reactions parallel biological reactions.
- Industrial Chemistry: Formate esters are also used in perfume and flavoring and plastics. Knowing how they react to water makes their storage and use right.
- Environmental Science: Because of the degradation trail of esters, learning about hcochron Ul:han (ch2omeztoiy2o) can be beneficial to understanding how they break down in aqueous settings, which is crucial when it comes to environmental control as well as making waste disposal.
Hydrolysis of Esters: Reaction Perspective:
Among the clearest meanings ofhcoech2 h2o is the hydrolysis of a formate ester. Simply stated:
Ester + Water ->Acid + Alcohol
In this case, the ester group HCOOCH gets reacted with H2O to give the formic acid (HCOOH) and an alcohol (based on the framework of the CH2 group). It is a typical scenario in organic chemistry and gives routes toward the formation of useful compounds.
Uses of Reactions With hcooch ch2 h2o
- Synthetic Chemistry: Careful Hydrolysis assists in the generation of non-acidic organic acids and alcohols which can be used as synthetic precursors.
- Food and Flavoring Industry: Food flavoring is frequently made of esters. Their controlled decomposition in the form of hcoech 2 h 2 o determines flavour stability.
- Polymer Science: Esters: Ester based polymers (polyesters) are subject to hydrolysis. Their activity with water will enable recyclability and durability.
- Medical Chemistry: To guarantee the possibility of bioavailability and metabolism of ester containing pharmaceuticals hydrolytic reactions find application in drug design.
Safety and Handling
Hcooch ch2 h2o related chemicals need some care:
- Esters may be flammable as well as volatile.
- A hydrolysis product is formic acid which is corrosive and necessitates the use of adequate protection.
- Reactions with water are to be watched not to form uncontrolled hydrolysis, especially on sensitive compounds.
Lab procedures also make reactions with hcooch ch2 h2o safe and give precise results.
Conclusion
hcooch ch2 h2o is not just a convenient chemical shorthand; the notation expresses the fundamental ideas of ester chemistry and hydrolysis, and of organic reactivity. Examining its constituents, formate esters, methylene groups and water, we reveal its usefulness in pharmaceuticals, industry, the environmental science and synthetic chemistry. Learning and knowing these modes of chemical notations will assist researchers, students, and professionals to simply understand how little groups of molecules conspire to create different reactions to both natural and industrial processes.
Finally, hcooch ch2 h2o is one of the most important illustrations of the ways how chemistry connects sets of simple groups into comprehensible changes, contributing to innovation in various scientific spheres.